Intro:
Modern food manufacturing facilities now employ many types of automated processing equipment with computerized control systems. This article will cover the automated systems controlling the commercial sterilization of human and pet food in hermetically sealed containers. Due to the automated nature of these control systems and the various critical process factors they control, various safeguards must be in place to ensure that the process, and therefore the safety of the product, is not compromised. Additionally, there must be a system in place to effectively record or document, and in many cases, validate each critical process component according to existing regulations.
Capabilities
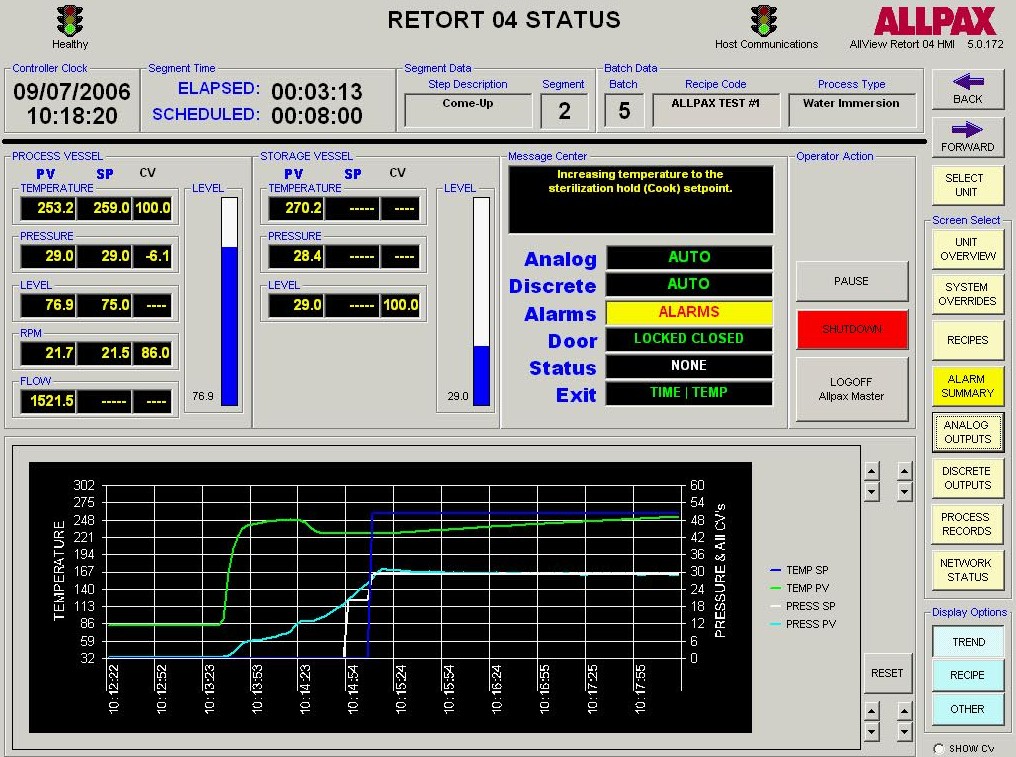
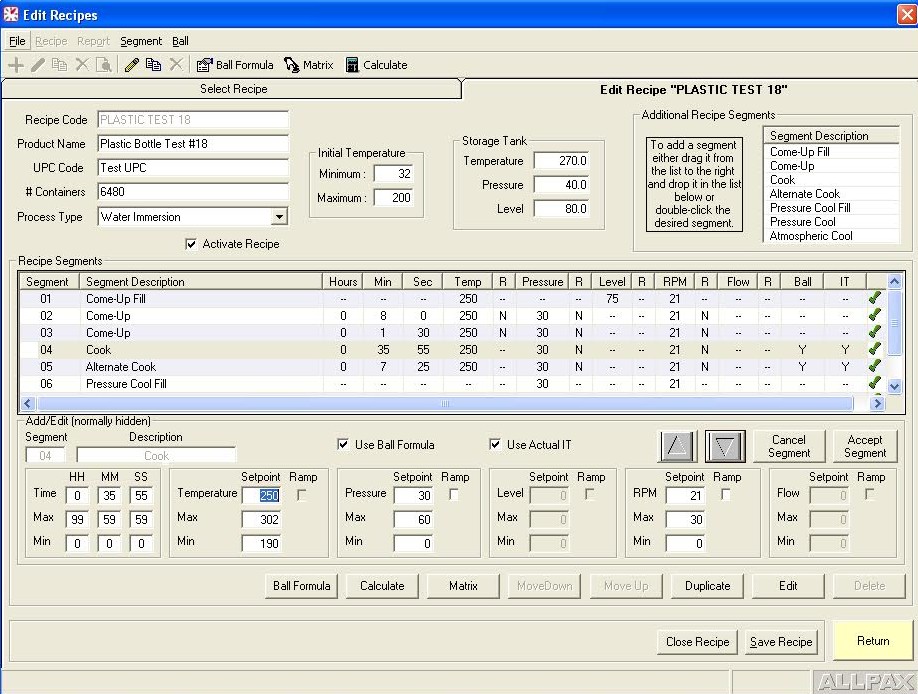
Retort process control and recording software falls into two categories: Human–Machine Interface (HMI) control software and Host software. The HMI software allows tracking of operator actions, inputting allowed operator privileges and allowed retort step overrides, manually overriding discrete and analog outputs such as valves & motors, tuning valve control loops for optimal performance, live viewing of retort logs and trends as well as accessing current and archived alarms. The Host program is the software interface to the control program which serves several functions. It is where process step parameters (recipes) are configured and where supervisors can assign operator privileges and securities. The host software also interfaces with the control software’s logging engine to generate various process reports including alarm reports, trend reports and many other configurable report formats based on critical factors of the automated process. Many of these reports are federally required or internally mandated by food manufacturers in order to prevent and mitigate food chain hazards and costly downstream issues such as product recalls and the associated brand name damage.
In addition to control and reporting of the processing of commercially sterilized products, material handling is also a primary consideration of the production process as it affords multiple opportunities for unprocessed or under processed product to inadvertently be introduced into the distribution stream. In completely automated systems (ABRS), these safeguards are accomplished with photoelectric sensors to track the flow of unprocessed/under processed and processed product and input these data into the programmable logic controllers where throughput safeguards are in place to prevent retort bypass hazards. For effective monitoring of product throughput in manual material handling applications, similar considerations need to be made to prevent the improper handling of food products by human operators. This usually requires the installation of handheld and machine mounted scanners that record the progress of a designated lot of product through the retort room production process.
Compliance
In the United States, food production for human and pet consumption fall under the regulatory purview of either the Food and Drug Administration (FDA) or the United States Department of Agriculture (USDA). Under whichever purview of these federal departments a particular product falls under is another discussion altogether. Regardless of such, the operating software and the corresponding records keeping software are regulated under Title 21 of the Federal Code of Regulations Part 11. In addition to a myriad of other applications, 21CFR P11, for short, establishes the requirements for any electronic records keeping, process reporting and electronic signatures protocol that pertains to commercially sterilized products. Effective reporting software is capable of documenting every single process input and output, every human input into the HMI and host programs as well as every process parameter and trend all, while establishing a documentable chain of ownership of all process parameters and operator inputs and or overrides.
Validation
Retort room validation encompasses the most intricate and thorough aspect of hazard prevention a manufacturer of commercially sterilized food products can employ. It requires many hours of a software engineer manually forcing every single input and output of an intricate, fully automated, multi-machine system and ensuring that all recording reporting and alarm generating protocols as well as alternate process protocols are functioning properly under all possible conditions. Due to the breadth of this process and the associated software programs that are employed to facilitate the ongoing validation of a retort room, we will dive deeper into this topic in one of our upcoming white papers here on Retorts.com.
